Home » Industries » Life Sciences » How digital tech enables virtual clinical trials
Game changer IoT
In 2017, General Electric’s device for taking data related to the bloodwork in an ongoing trial and sharing it securely with the trial sponsor built confidence in virtual collection of information. The need to meet candidates for a physical checkup, get their bloodwork done and move on to collect medication was cost and labor-intensive. With smartphones, apps and fitness trackers measuring these parameters virtually, delivering the medications to candidates became easier.
The rise of social media
Social media makes it quicker and simpler to find the right matches for clinical trials. Finding people with specific medical conditions just got easier. It is brisker to sift through smaller groups of thousands rather than millions, as also to connect with them once the trials are underway through the same platforms. Connecting sponsor records to the interactions via social media was a game changer in the recruitment process.
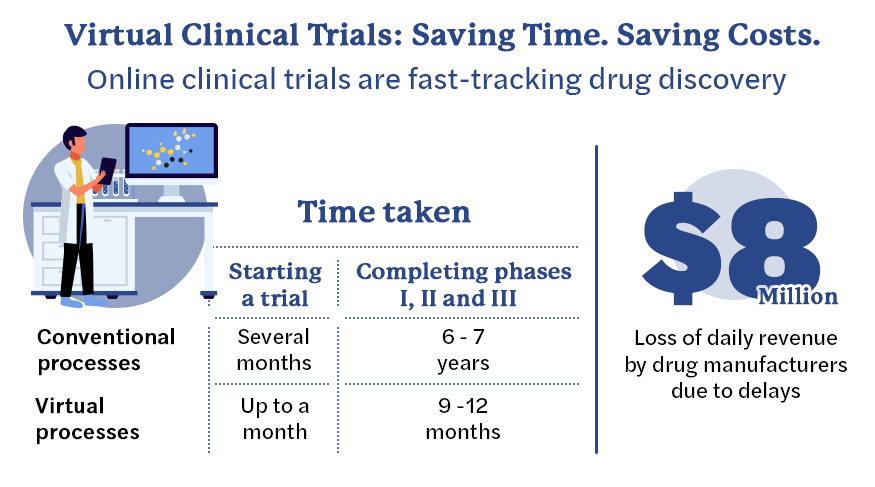
Advantages of virtualization
Conventionally, getting a trial started would take months, if not years. With virtualization of the processes and experienced teams managing them, that can take just a month. The speed of setting up a trial, administering it, collecting the data and reporting it has been reduced significantly. Phases I, II and III of clinical trials can now be completed in nine to 12 months – they used to take six to seven years earlier. Updating the trials’ database for the U.S. Food and Drug Administration and regulatory bodies elsewhere on an almost real-time basis will soon be an area of focus, as virtual clinical trials help organizations speed up the process and deliver results in record time.
Virtual clinical trials go viral
Pharmaceutical giant Novartis has begun testing its experimental drugs on a digital platform. Virtual clinical trials are being conducted for medication to treat cluster headaches and NASH syndrome, a non-alcoholic fatty liver disease. These are in addition to their clinical trials for drugs to treat COVID-19.
The first virtual clinical trials were conducted by Pfizer in 2011 for the development and use of wearables in phase three of Parkinson’s disease. They continue to innovate in the use of wearables – with explorations into sleep patterns being affected by chronic skin conditions – that allow them to track patients beyond a lab or testing facility and into the real world.
No turning back
The pandemic has forced everyone in different stages of the system to adapt rapidly. Stakeholders across the value chain must re-evaluate their business models and adapt to technology. Anti-coronavirus vaccines will be able to go through all trial stages within a year; such speed would have been unthinkable in the past.
Key takeaways
- COVID-19 prevention norms and vaccine urgency boost virtual clinical trials.
- Social media quickly takes the method far and wide, proving to be a game changer.
- Time and money saved by switching from traditional methods are phenomenal.
- Online trials simplify and speed up the process, cutting it from a year to a month in some cases.
- Most pharma majors have opted for online clinical trials. Virtual clinical trials have now become the first resort.
Contact
Our experts can help you find the right solutions to meet your needs.
Get in touch
